
|
Photophysics and Photochemistry of Transition Metal Compounds |
| Home Research Members Collaborations Publications |

|
 |
|||||||
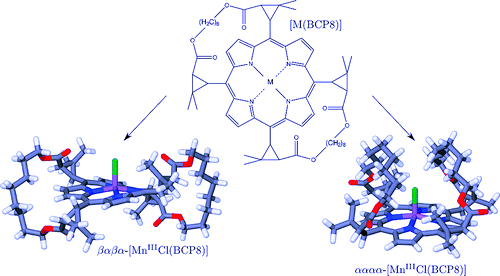
Transition metal complexes of chiroporphyrins, in which two adjacent meso substituents are linked by a strap of eightmethylene groups, [M(BCP8)], can exist as either an αααα or αβαβ atropisomer depending on the nature of thecoordinated metal cation. This remarkable conformational versatility was investigated by density-functional theorycalculations for the d5 chloroiron(III) complex in the low-spin and high-spin states and for the d4 high-spinchloromanganese(III) complex. The lowest-lying electronic state of all of the conformers of the chloroiron(III) bridledchiroporphyrin is found to be the high-spin state. For the chloroiron(III) complex in the low-spin or the high-spin stateand for the high-spin chloromanganese(III) complex, the most stable form is predicted to be the αααα conformer inwhich the chloride axial ligand is located within the cavity provided by the bridles. The predicted stereochemistries arecompared with those similarly obtained (i) for the chloroiron(III) and chloromanganese(III) complexes of thetetramethylchiroporphyrin, which is devoid of straps, and (ii) for the d10 zinc(II) and low-spin d8 nickel(II) BCP8complexes, on the basis of the effects tied to the occupancy of the stereochemically active dx2-y2-type antibondingorbital level, to the restraints imposed by the straps, and to the presence of the axial chloride ligand. | ||||||||
|
||||||||
Circular dichroism (CD) spectra and density functional theory (DFT) calculations are reported for a series of conformationally bistable chiroporphyrins with 8-methylene bridles MBCP-8, which can display either an αααα or an αβαβ orientation of their meso substituents. From DFT geometry optimizations, the most stable form of ZnBCP-8 is found to be the αααα conformer. By passing to NiBCP-8, there is a strong stabilization of the αβαβ conformation with respect to the αααα conformation, consistent with the X-ray structures of αααα-ZnBCP-8 and αβαβ-NiBCP-8. A correlation between the sign of the CD signal in the Soret region and the conformation of the BCP-8 compounds is reported: the αααα conformers H2BCP-8 and ZnBCP-8 show a positive CD signal, whereas the αβαβ conformers NiBCP-8 and CuBCP-8 exhibit a negative signal. The possible contributions to the rotational strengths of αβαβ-NiBCP-8 and αααα-ZnBCP-8, calculated on the basis of their crystal structures, have been analyzed. The CD signals are found to result from a combination of both the inherent chirality of the porphyrin and of extrinsic contributions due to the chiral bridles. These results may have a broad significance for understanding the chiroptical properties of chiral porphyrins and hemoproteins and for monitoring stimuli-responsive, conformationally bistable chiroporphyrin compounds. | ||||||||
Download this list in format RIS
 EndNote
EndNote  BibTex
BibTex  PDF XML
PDF XML Last update Friday December 08 2017